Background:Cold agglutinin disease (CAD) is a rare, chronic autoimmune hemolytic anemia characterized by complement-mediated hemolysis. The classical complement inhibitor sutimlimab, is the first approved pharmacotherapy for treating patients with CAD. The second-generation complement inhibitor SAR445088 (BIVV020) is a humanized monoclonal antibody that selectively inhibits the activated form of C1s (sutimlimab inhibits total C1s). Furthermore, SAR445088 contains mutations that increase FcRn binding, preventing its degradation and allowing it to be recycled back into the system, resulting in reduced drug clearance and a prolonged half-life, which may allow a longer dosing interval than that of sutimlimab.
Aims:This Phase 1b open-label study evaluated the safety, tolerability, effect on hemolysis, and pharmacodynamics (PD)/pharmacokinetics (PK) of a single intravenous (IV) dose of SAR445088. The study aimed to assess the impact of SAR445088 on complement-mediated hemolysis to support proof of concept for SAR445088 as a treatment for patients with CAD.
Methods:The study design included up to three single-dose cohorts with a maximum of six patients per cohort. SAR445088 was administered on Day 1 with follow-up visits from Day 2 to Day 71; end of study (EOS) visit was on Day 106. The first cohort received a dose of 30 mg/kg IV with decisions about the selection of the dose for the next cohort being based on safety, biomarker activity, and PD. In the second cohort, patients received a lower dose of 15 mg/kg IV; a third, higher-dose cohort was not enrolled. To be enrolled in the study, participants needed to be aged ≥18 years with a confirmed diagnosis of CAD and a hemoglobin level ≤11.0 g/dL at time of screening. The primary endpoint was assessment of safety, with secondary endpoints of hematologic biomarkers and PD/PK.
Results:Twelve patients received a single IV dose of SAR445088 (n=6, 30 mg/kg; n=6, 15 mg/kg). Patients enrolled in the study were mainly female (91.7%, n=11) with a median age (range) of 69 (54-80) years, and median (range) hemoglobin at baseline was 9.60 (4.8-10.9) g/dL. There were no treatment-emergent serious adverse events (AEs), discontinuations or deaths reported in the study. Fifty-eight treatment-emergent AEs (TEAEs) were reported in 11 patients (91.7%). There was one reported pre-treatment severe AE (Grade 3) of hemolytic anemia in a patient in the 15 mg/kg IV cohort which began prior to treatment and continued during the treatment period; the remainder of TEAEs were mild or moderate in severity (Grade 1 or 2). The most frequently reported AE was hematuria. No serious infections, meningococcal infections, hypersensitivity, or thromboembolic events were reported.
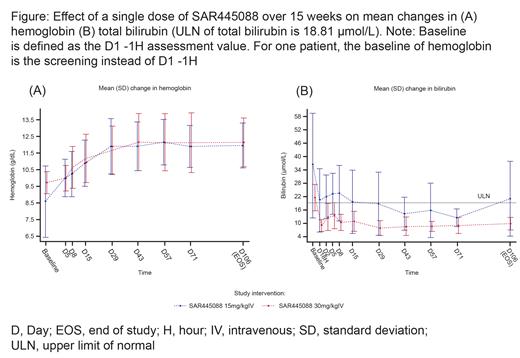
Nine patients (n=4, 30 mg/kg IV cohort; n=5, 15 mg/kg IV cohort) reached an increase of hemoglobin ≥1.5 g/dL from baseline to Day 106 (EOS visit); eight patients (n=4 for both cohorts) reached an increase in hemoglobin >1.0 g/dL from baseline to Day 15 (Figure A). Total mean bilirubin decreased by Day 1 and this was sustained to Day 106 (EOS visit; Figure B).The decrease was more pronounced in the 30 mg/kg IV cohort. Improvements in clinical markers correlated with sustained reductions in CH50 in both treatment cohorts. Reductions in mean percent classical complement pathway (CP) activity were noted 1 hour after a single dose of 30 or 15 mg/kg IV, followed by gradual rebound of CP activity over the 15-week study period.
Conclusion:SAR445088 was generally well tolerated; no safety concerns were identified in patients with CAD. A single IV dose of SAR445088 led to classical complement inhibition, control of hemolysis, and improvement in anemia, which was sustained for 15 weeks.
OffLabel Disclosure:
D'Sa:BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite: Honoraria; Janssen: Honoraria. Vos:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Research Funding; Abbvie: Research Funding; Genmab: Research Funding; BMS: Speakers Bureau; Amgen: Speakers Bureau. Barcellini:Novartis: Consultancy, Honoraria, Speakers Bureau; Alexion, AstraZeneca Rare Disease: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wardęcki:Sanofi: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Perrin:Sanofi: Current Employment, Current equity holder in publicly-traded company. Barker:Trizell Ltd: Consultancy, Current Employment. Zilberstein:Sanofi: Current Employment, Current equity holder in publicly-traded company. Storek:Sanofi: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties; Q32 Bio: Current holder of stock options in a privately-held company, Patents & Royalties. Chow:Sanofi: Current Employment, Patents & Royalties. Roeth:Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Biocryst: Consultancy, Honoraria; Apellis Apellis Pharmaceuticals: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Bioverativ: Consultancy, Honoraria; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria.
SAR445088 (BIVV020) is a second-generation classical complement inhibitor for the treatment of patients with CAD. Its mechanism of action targets only the activated form of C1s, potentially enabling longer dosing intervals due to reduced clearance and a prolonged half-life compared to the approved sutimlimab. The resulting more favorable pharmacokinetic profile may reduce dosing frequency, ease healthcare utilization costs, reduce patient burden and increase compliance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal